
Home List your patent My account Help Support us
A Medical Device For Treating Obstructive Sleep Apnea and Snoring
[Category : - HEALTH]
[Viewed 3437 times]
Patent: US 8,739,794
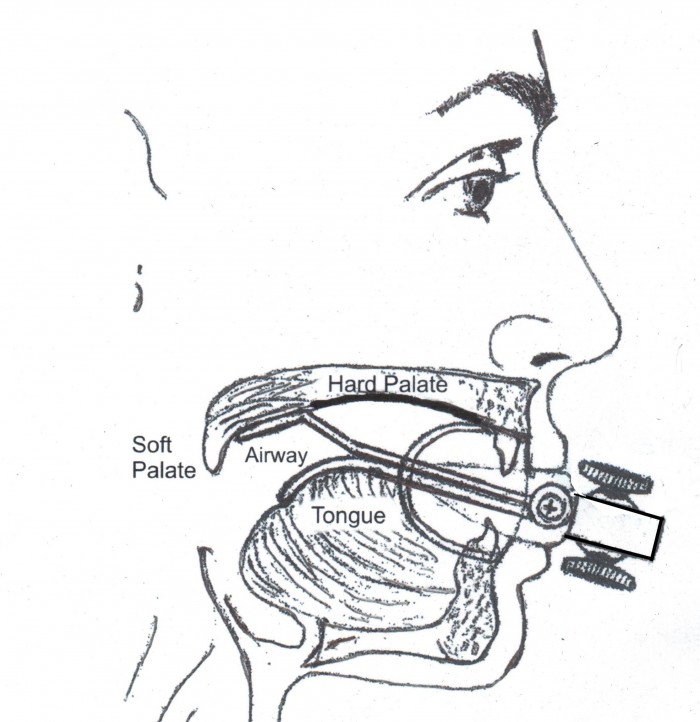
The patent describes a hybrid device to treat Obstructive Sleep Apnea. It consists of a soft palate supporter and a tongue retainer anchored in a device body and held in place by a soft silicone mouthpiece. Both the soft palate supporter and the tongue retainer are individually adjustable in the length to which they can protrude into the oral cavity so that they can be adjusted to the optimum length by users of various sizes.In limited testing, the device has reduced the users AHI(Apnea/Hypopnia Index) from "severe" to within normal limits.
The device can be adjusted by a physician, or by the end user with detailed instructions. Although it creates some initial discomfort for some users(as would be expected with a device designed to alter the physiology within the oral cavity), it is no more difficult to use than CPAP or other intra oral devices. The majority of potential users can overcome this discomfort with instructions available over the internet, or with the aid of a physician.
The device can be mass produced and sold for a fraction of the cost of existing technologies in some countries and with FDA approval in the USA.
Financial information
I am looking for a strategic partner, preferably a physician, to help finance the FDA approval process and design enhancements which improve the efficacy of the device and simplify production of the device and maintenance of the device by the user. Partnership details negotiable.
I am also willing to sell the patent outright for $95,000.
The device was sold on a very limited basis in Canada, Australia, and New Zealand. The goal was to determine acceptance of the device across different markets and to quantify that acceptance using a 30 day money back guarantee, Approximately 72% acceptance was realized, well above the 55-60% acceptance of CPAP, considered the gold standard of sleep apnea treatments. This compliance was generated without he aid of physicians, and can be increased if a medical professional is involved.
Global changes in the licensing of medical devices has raised questions about legality of marketing in foreign markets, so marketing of the device has been suspended pending FDA approval.
The device can be viewed at: Link where a detailed description of the device, test results, physician testimonials, and user testimonials are available.
The patent can be viewed at: Link
Also read the article in the Sleep and Health Journal:
Link
Asking price:
95,000 USD
95,000 USD




[ Home | List a patent | Manage your account | F.A.Q.|Terms of use | Contact us]
Copyright PatentAuction.com 2004-2017
Page created at 2025-09-16 5:17:20, Patent Auction Time.
 Patent publications:
Patent publications: US 8739794
US 8739794 Great invention
Great invention